Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ichihara, Akira; Abe, Yutaka*
no journal, ,
Light and heavy water are being used as moderators in fission reactors. Reliable data of thermal neutron scattering in these materials is essential to the analysis of reactor cores. In this study we tried to evaluate angular distribution and energy spectrum of emitted neutrons employing the information obtained from molecular dynamics simulations. The simulations have been performed for light and heavy water. The thermal neutron scattering has been evaluated by using the fluctuation-dissipation theorem of statistical mechanics, where the trajectory data for hydrogen, deuterium and oxygen atoms were utilized. In calculation, incoherent scattering was assumed to be dominant in light water, while both the coherent and incoherent scattering contributions were considered in heavy water.
Sekine, Yurina; Nankawa, Takuya; Miura, Daisuke*; Yunoki, Shunji*; Sugita, Tsuyoshi; Nakagawa, Hiroshi; Yamada, Teppei*
no journal, ,
We developed a crosslinking method using freeze concentration and used it to synthesize a cellulose nanofiber hydrogel with high compressive strength. The reaction between the freeze-concentrated cellulose nanofiber and citric acid created a rigid porous structure. Without the freeze crosslinking method, the complex of the cellulose nanofiber sol and citric acid produced hydrogels, which easily collapsed under compressive stress. We will give a presentation on the crosslinking method and the chemical and physical properties of the hydrogel.
Ouchi, Kazuki; Muto, Yuki*; Brandt, A.*; Nabatame, Nozomi*; Tsukahara, Takehiko*; Kitatsuji, Yoshihiro
no journal, ,
The adsorption and elution performance of uranium with the microchip column (length 11 mm, column volume 0.39  L) loaded an anion exchange resin for faster and safer radioactive waste analysis were investigated. When a mixed sample of lanthanides and uranium was flown on the microchip column at the feed rate of 1 ml h
L) loaded an anion exchange resin for faster and safer radioactive waste analysis were investigated. When a mixed sample of lanthanides and uranium was flown on the microchip column at the feed rate of 1 ml h , uranium could be selectively adsorbed and eluted in about 4 minutes of operation time. This was faster than a conventional column which took several tens of minutes. When this column was applied to uranium separation of the standard seawater, the uranium concentration was determined to be 2.86
, uranium could be selectively adsorbed and eluted in about 4 minutes of operation time. This was faster than a conventional column which took several tens of minutes. When this column was applied to uranium separation of the standard seawater, the uranium concentration was determined to be 2.86 0.05 ppb, which was in good agreement with the certified value (2.81
0.05 ppb, which was in good agreement with the certified value (2.81 0.16 ppb). Therefore, the verification test was successful.
0.16 ppb). Therefore, the verification test was successful.
Okutsu, Kenichi*; Kino, Yasushi*; Nakashima, Ryota*; Miyashita, Konan*; Yasuda, Kazuhiro*; Yamashita, Takuma*; Okada, Shinji*; Sato, Motoyasu*; Oka, Toshitaka; Kawamura, Naritoshi*; et al.
no journal, ,
A muon is one of elementary particles which is known to weight 207 times more than an electron. A nuclear fusion reaction occurs in a muonic molecule which consists of two hydrogen isotope nuclei and a muon because the muon binds more tightly than electron. Since the muon does not directly participate in the fusion reaction, the reaction is called muon catalyzed fusion ( CF). The muon released after the reaction is called a "recycling muon", and maintains the molecular orbital information when the muonic molecule formed. Therefore, information of the muon wavefunction can be investigated by observing the energy distribution of the recycling muon. We will report the experimental setup for measuring the energy distribution of the recycling muons after the nuclear reaction.
CF). The muon released after the reaction is called a "recycling muon", and maintains the molecular orbital information when the muonic molecule formed. Therefore, information of the muon wavefunction can be investigated by observing the energy distribution of the recycling muon. We will report the experimental setup for measuring the energy distribution of the recycling muons after the nuclear reaction.
Miura, Daisuke; Nankawa, Takuya; Yamada, Teppei*; Sekine, Yurina
no journal, ,
We developed a crosslinking method using freeze concentration and used it to synthesize a carboxymethyl cellulose nanofiber (CMCF) hydrogel with high compressive strength and high formability. The reaction between freeze-concentrated CMCF and organic acids created a rigid porous structure. In this study, we found that the structure of the freeze-concentrated CMCF could be fixed by organic acids in presence of ice, and the fixed structure contributed the mechanical properties of the hydrogel. We will give a presentation on the gelation mechanism of the CMCF hydrogel.
Sato, Tetsuya; Nagame, Yuichiro*; Eichler, R.*
no journal, ,
Element 103, lawrencium (Lr) has been pointed out that it might have significantly high volatility more than that of lutetium (Lu), the lanthanide homolog of Lr, owing to an influence of the strong relativistic effect. In our previous work, we have measured the first ionization potential of Lr by using the surface ionization method. In the method, the Lr atom was surface ionized on a tantalum surface at a high temperature. If the surface temperature is sufficiently high, the ionization efficiency of Lr can be estimated by the Saha-Langmuir (S-L) equation. However, an adsorption loss of the atoms of interest onto the metal surface could be inevitable at a low temperature. In that case, the apparent ionization efficiency would become smaller than the value predicted from the S-L equation. We have developed a Monte Carlo simulation code to estimate the adsorption loss of atoms on the ion-source surface in this work. The simulation code describes the ionization behavior of the atoms by combining the thermal ionization process in the ionizer and the adsorption-desorption process on the surface.
Otani, Ryo; Sato, Tetsuya; Aoki, Ryota*; Shirai, Kaori*; Suzuki, Hayato; Tsukada, Kazuaki; Asai, Masato; Ito, Yuta; Nagame, Yuichiro*; Sakama, Minoru*
no journal, ,
The adsorption enthalpy of the Sg dioxydichloride on a quartz surface ( (SgO
(SgO Cl
Cl )) has been reported to be -98 kJ/mol. The result, however, is still ambiguous because the value was evaluated based on a few experimental points with large statistics errors. To obtain a reliable
)) has been reported to be -98 kJ/mol. The result, however, is still ambiguous because the value was evaluated based on a few experimental points with large statistics errors. To obtain a reliable  (SgO
(SgO Cl
Cl )) for a discussion of an influence of relativistic effects, a stable gas chemistry apparatus with good reproducibility is mandatory. In this study, we have conducted offline experiments of an isothermal gas chromatography) with short-lived Mo isotopes originated from a
)) for a discussion of an influence of relativistic effects, a stable gas chemistry apparatus with good reproducibility is mandatory. In this study, we have conducted offline experiments of an isothermal gas chromatography) with short-lived Mo isotopes originated from a 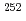 Cf fission source. We searched for experimental parameters for an on-line experiment. At the presentation, we will present the obtained optimum conditions for gas chromatographic separation of group-6 elements and determined
Cf fission source. We searched for experimental parameters for an on-line experiment. At the presentation, we will present the obtained optimum conditions for gas chromatographic separation of group-6 elements and determined  (MoO
(MoO Cl
Cl )).
)).
Aoki, Ryota*; Sato, Tetsuya; Otani, Ryo; Suzuki, Hayato; Ito, Yuta; Asai, Masato; Tsukada, Kazuaki; Nagame, Yuichiro*
no journal, ,
To apply a SHEs ion-beam to physical and chemical investigations, we have been developing ion sources that apply to a short-lived single atom. We employed the Electron Beam Generated Plasma (EBGP) method to ionize an element with high ionization energy. The EBGP ion source can ionize an element or a molecule of nuclear reaction products by bombarding an electron beam that accelerated between the cathode and the anode electrode. In this work, we built the EBGP ion source and searched for an optimum condition ionization of a single atom.